Life
Sciences
We provide timely and accurate drug development and commercialization services from our U.S. owned and operated CDMO/CRO network.
improving our health
We believe the therapies our customers develop are critical to improving lives and we are proud to be a part.
Frank Tagliaferri, Ph.D. Featured in Panel Session on Advancing Oral Drug Delivery Beyond Lipinski’s Rule of 5
Chief Scientific Officer of Pace® Life Sciences Joins Industry Leaders at Advancing Drug Development Forum (ADDF) to Collaborate on Technologies for Non-Traditional Molecules
Pace® Life Sciences to Hold Free Virtual Webinar through Outsourced Pharma on USP Guidelines for Extractable and Leachable Manufacturing Risk Assessment
Pace® Life Sciences is hosting a free virtual webinar providing an overview of the USP requirements for extractable and leachable risk assessments for pharmaceutical and biopharmaceutical processes.
Pace® Life Sciences’ San German Site Receives Positive Inspection from FDA
Pace® Life Sciences announced today that its San German, Puerto Rico site has received a positive FDA Inspection of its quality systems and client data delivery processes.
Pace® Life Sciences has been recognized by PharmaSource as a Top CDMO in the United States
PharmaSource has recognized Pace® Life Sciences as a Top CDMO in the United States, noting our comprehensive solutions, strong regulatory track record, and customer-focused approach!
Pace® Life Sciences Debuts Enhanced cGMP Manufacturing Capabilities to Support Increasing Demand for Sterile Injectables
Dawn Von Rohr, President of Pace® Life Sciences, stated, “This investment reflects our unwavering commitment to our partners, as we work towards accelerating the delivery of medicines into the clinical setting faster….”
Sterile-Fill Finish Manufacturing Expansion
The demand for fill-finish manufacturing continues to grow due to the rising number of biologics and gene therapy products being developed, predominantly for parenteral administration. The increasing introduction of more convenient and user-friendly routes of administration for these products has also significantly contributed to the demand for more manufacturing capacity.
Pace® Life Sciences recognizes the underlying market drivers influencing our clients’ advancements, which informs our latest investments to expand sterile filling contract manufacturing capabilities in Salem, NH. Explore the official debut of our Sterile Fill-Finish Center of Excellence. We look forward to helping advance your program to the next phase.
Explore our range of services
Wherever you are in your drug development journey, we can help.
THE RIGHT PARTNER FOR YOUR PROJECT
We have a nationwide network of state-of-the-art facilities, each with long-established histories of successful product development and commercialization and excellent audit outcomes from regulatory agency and client reviews.
FDA
REGISTERED
DEA
DEA Schedules I – V
GMP
COMPLIANT
ISO
17025 ACCREDITED
OUR PROMISE TO YOU
We honor our commitments so you can honor yours™. Our investment in state-of-the-art facilities and highly trained experts emphasizes our commitment to delivering positive customer experiences across all phases of pharmaceutical and biopharmaceutical development.
- RELIABLE DELIVERY
- COLLABORATIVE RELATIONSHIPS
- EXCEPTIONAL SERVICE
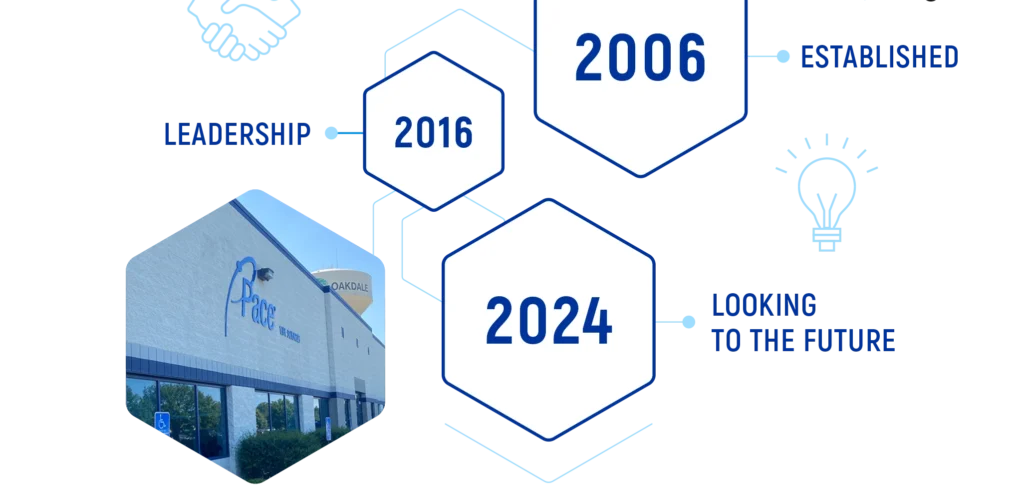
IT ALL STARTED IN 2006
Since 2006, Pace® Life Sciences has continued to prioritize strategic investments and domestic acquisitions to meet the changing needs of our customers. As the market changes, we are committed to making sure we are positioned as the best U.S. owned and operated end-to-end solution for your program.
Conferences

DCAT Week
The Premier Event for the Global
Bio/Pharmaceutical Business Ecosystem
March 23-26, 2026 | New York City
feature webinar

Overview of FDA’s Expedited Programs for Serious Condition
Recognize the importance of expedited review programs, such as FTD, BTD, and RMAT Designation, and their impact on reducing drug development time.
Now available on demand