*Required field: Failure to fill in a required field may result in a sample(s) being put on hold until information can be obtained. This may result in a delay in receiving results.
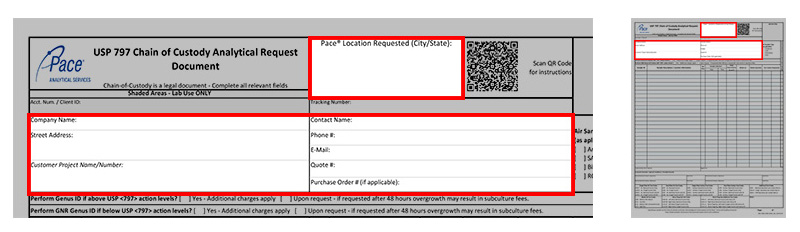
*Pace Location Requested: City and State of Pace Laboratory where testing is to be performed. Click Here for Pace® Lab Locations
*Company Name: Client’s company name
*Street Address: Client’s mailing address, city, state, and zip code for mailing
Customer Project # and Project Name/Description: Client’s reference to the project or work involved with these samples.
*Contact Name: Person to receive results
*Phone #: Client’s contact phone number
E-mail: Client’s e-mail for correspondence
Quote #: Client or project specific number for client billing purposes.
Purchase Order #: Client specific number to be listed on project invoice for client billing purposes.
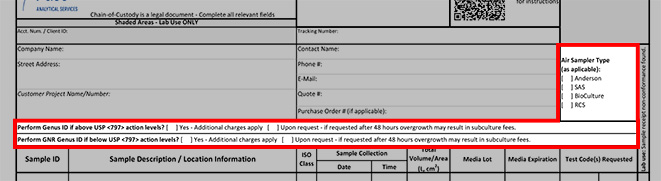
*Air Sampler: Required for air monitoring, select the type of sampling equipment used.
Perform Genus ID if above USP <797> action levels: Select Yes or No. Additional charges may apply.
Perform GNR Genus ID if below USP <797> action levels: Select Yes or No. Additional charges may apply.
*Sample ID: The unique sample ID you want to appear on the analytical report
*Sample Description / Location Information: Describe the sample and/or location
*ISO Class: Provide the air-quality classification from the International Organization for Standardization for each area monitored.
*Collected Date: Date sample was collected.
*Collected Time: Time sample was collected.
*Total Volume/Area: Where applicable, record the total volume of air sampled in liters (L) or the total surface area sampled in square centimeters (cm2).
*Media Lot: Where applicable, record the lot of media used for collection.
*Media Expiration: Where applicable, record the expiration date of the media used for collection.
*Analysis/Test Code Requested: Fill-in the test codes for the desired analysis for each sample.

Single Plate Air Test Codes
1202.7 Air, Bacterial Counts Only
1202.8 Air, Fungal Counts Only
1202.5 Air, Bacterial Counts with ID
1202.6 Air, Fungal Counts with ID
Dual Plate Air Test Codes
1207 Air, Bacterial Counts
1208 Air, Fungal Counts
1107 Air, Bacterial Counts with ID
1108 Air, Fungal Counts with ID
Single Plate Surface Test Codes
1202 Surface, Bacterial Counts Only
1202.1 Surface, Fungal Counts Only
1202.2 Surface, Bacterial Counts with ID
1202.3 Surface, Fungal Counts with ID
Dual Plate Surface Test Codes
1204 Surface, Bacterial Counts Only
1206 Surface, Fungal Counts Only
1104 Surface, Bacterial Counts with ID
1106 Surface, Fungal Counts with ID
Additional Test Codes
1064 Bacterial Speciation per Isolate Add-on
1064.4 Fungal Speciation per Isolate Add-on
1064.1 GNR Speciation per Isolate Add-on
Media Fill Test Codes
1209 Medium Risk (Basic)
1211 Low Risk
1212 Medium Risk (Comprehensive)
1213 High Risk
Glove Fingertip Test Codes
1109-LB Left Hand, Bacterial Counts Only
1109-RB Right Hand, Bacterial Counts Only
1110-LF Left Hand, Fungal Counts Only
1110-RF Right Hand, Fungal Counts Only
Glove Fingertip Additional Test Codes
1109.1-L Left Hand, Bacterial Counts with Genus ID
1109.1-R Right Hand, Bacterial Counts with Genus ID
1110.1-L Glove Fingertip, Left Hand, Fungal Counts with Genus ID
1110.1-R Glove Fingertip, Right Hand, Fungal Counts with Genus ID
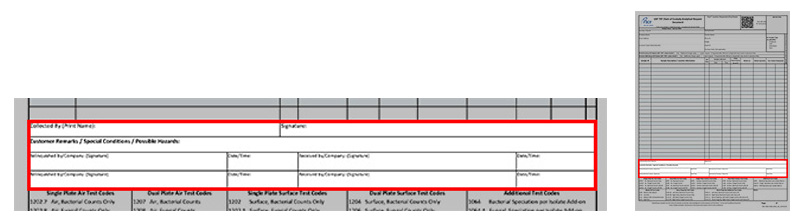
*Collected By: Printed name of sample collector
*Collected By Signature: Signature of sample collector
Customer Remarks/Special Conditions/Possible Hazards: List special instructions about the sample here. If the sample is known or suspected to be hazardous indicate that here and attach SDS if possible.
*Relinquished By/Received By: This form must be signed each time the sample(s) changes hands. Custody seals are available upon request if needed.
Summarized Sample Acceptance Policy Requirements:
- Proper, full, and completed chain-of-custody documentation
- Legible unique sample container identification written in indelible ink
- Appropriate sample container
- Enough sample to perform the requested tests
- Received within required holding time, where applicable
- Received within temperature preservation requirements, when necessary
- Sample containers received in good condition (not leaking or broken)
- Custody seals, when used, are intact
- Properly preserved, when required
A data qualifier and/or case narrative will be added to the final test report when the above sample acceptance requirements are not met. Full location Specific Sample Acceptance Policy is available from your Project Manager.
.